Understanding Trigonal Pyramidal Geometry: A Comprehensive Guide
Trigonal pyramidal geometry is a fundamental concept in chemistry that describes the shape of molecules with four electron pairs around a central atom, where one of the pairs is a lone pair. This molecular geometry plays a crucial role in determining the physical and chemical properties of various substances. Understanding its principles allows chemists and students to predict how molecules behave in different environments. This article provides an in-depth exploration of trigonal pyramidal geometry, its characteristics, and its applications in real-world scenarios.
Whether you're a student studying chemistry or a professional seeking to expand your knowledge, this comprehensive guide will walk you through everything you need to know about trigonal pyramidal geometry. From its definition and structure to its implications in molecular interactions, we'll cover it all.
This guide is designed to ensure you gain a thorough understanding of the topic, complete with examples, diagrams, and references to credible sources. Let's dive into the world of molecular geometry and uncover the intricacies of trigonal pyramidal structures.
Read also:Celine Dion Funeral A Celebration Of Life And Legacy
Table of Contents:
- Definition and Basics of Trigonal Pyramidal Geometry
- Characteristics of Trigonal Pyramidal Molecules
- Examples of Trigonal Pyramidal Molecules
- Understanding Bond Angles in Trigonal Pyramidal Geometry
- The Role of VSEPR Theory in Predicting Trigonal Pyramidal Shapes
- Real-World Applications of Trigonal Pyramidal Geometry
- Comparison with Other Molecular Geometries
- Factors Influencing Trigonal Pyramidal Geometry
- Experimental Techniques for Identifying Trigonal Pyramidal Structures
- Conclusion and Next Steps
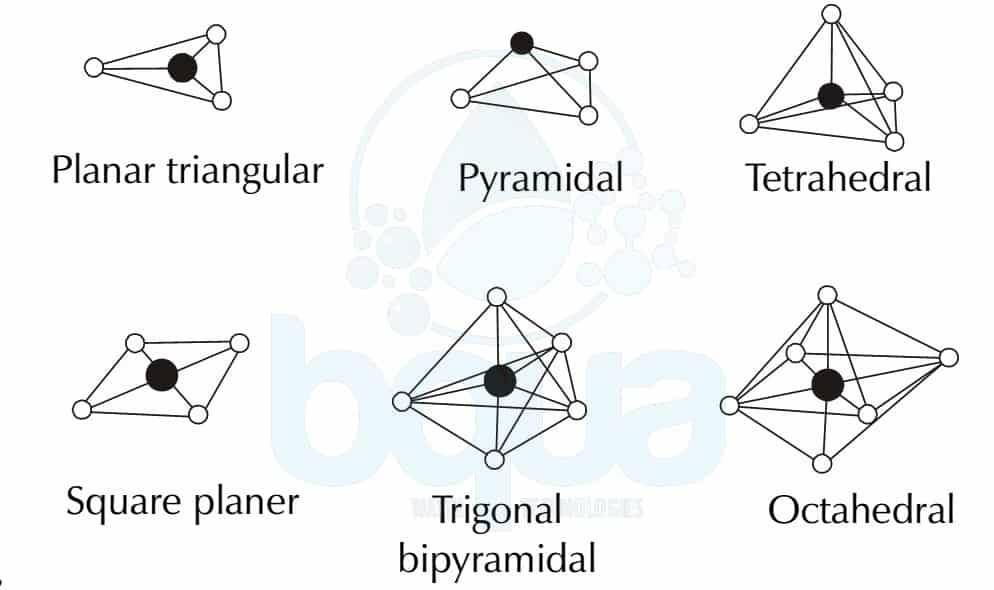
Definition and Basics of Trigonal Pyramidal Geometry
Trigonal pyramidal geometry refers to a molecular shape where a central atom is bonded to three other atoms and possesses one lone pair of electrons. This lone pair creates an asymmetrical distribution of electron density, leading to a pyramidal shape. The geometry is commonly observed in molecules like ammonia (NH₃) and phosphine (PH₃).
Key Features of Trigonal Pyramidal Geometry
- Central atom bonded to three surrounding atoms.
- One lone pair of electrons on the central atom.
- Asymmetrical electron distribution resulting in a pyramidal shape.
This geometry is critical in understanding molecular interactions, such as polarity and reactivity. By examining the arrangement of atoms and lone pairs, chemists can predict how molecules will behave in chemical reactions.
Characteristics of Trigonal Pyramidal Molecules
Trigonal pyramidal molecules exhibit distinct characteristics that differentiate them from other molecular geometries. These include bond angles, molecular polarity, and spatial arrangement.
Bond Angles in Trigonal Pyramidal Molecules
The bond angles in trigonal pyramidal molecules are typically around 107°, slightly less than the ideal tetrahedral angle of 109.5°. This deviation is due to the repulsion between the lone pair and bonding pairs of electrons.
For example, in ammonia (NH₃), the bond angle is approximately 107°. This reduction in angle is a direct result of the lone pair's greater electron density, which causes stronger repulsion compared to bonding pairs.
Read also:Haikyuu Battle Of The Garbage Dump The Ultimate Guide To One Of The Most Iconic Matches
Examples of Trigonal Pyramidal Molecules
Several common molecules exhibit trigonal pyramidal geometry. Here are a few examples:
- Ammonia (NH₃): A central nitrogen atom bonded to three hydrogen atoms with one lone pair.
- Phosphine (PH₃): Similar to ammonia, with a phosphorus atom replacing nitrogen.
- Chloramine (NH₂Cl): A derivative of ammonia where one hydrogen atom is replaced by chlorine.
These molecules are widely studied due to their importance in industrial and biological processes.
Understanding Bond Angles in Trigonal Pyramidal Geometry
Bond angles are crucial in determining the shape and properties of molecules. In trigonal pyramidal geometry, the bond angle is influenced by the repulsion between electron pairs. According to the Valence Shell Electron Pair Repulsion (VSEPR) theory, lone pairs occupy more space than bonding pairs, leading to a compressed bond angle.
Factors Affecting Bond Angles
- Electron pair repulsion: Lone pairs exert greater repulsion than bonding pairs.
- Atomic size: Larger central atoms can accommodate more electron pairs, affecting bond angles.
- Molecular environment: Surrounding atoms and substituents can influence the geometry.
Understanding these factors helps chemists predict and explain the behavior of trigonal pyramidal molecules.
The Role of VSEPR Theory in Predicting Trigonal Pyramidal Shapes
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a powerful tool for predicting molecular geometry. It states that electron pairs around a central atom will arrange themselves to minimize repulsion, resulting in specific shapes.
In the case of trigonal pyramidal geometry, VSEPR predicts that the lone pair will occupy a position that maximizes its distance from bonding pairs, leading to the characteristic pyramidal shape. This theory is supported by experimental evidence and is widely used in chemistry education and research.
Real-World Applications of Trigonal Pyramidal Geometry
Trigonal pyramidal geometry has numerous applications in various fields, including:
- Industrial Chemistry: Ammonia is a key component in fertilizer production and industrial processes.
- Biochemistry: Trigonal pyramidal molecules play a role in enzyme activity and biological processes.
- Environmental Science: Understanding molecular geometry helps in studying atmospheric pollutants like ammonia.
These applications highlight the importance of trigonal pyramidal geometry in both theoretical and practical contexts.
Comparison with Other Molecular Geometries
Trigonal pyramidal geometry differs from other molecular geometries in several ways:
Comparison with Tetrahedral Geometry
- Tetrahedral geometry involves four bonding pairs with no lone pairs, resulting in a symmetrical shape.
- Trigonal pyramidal geometry has one lone pair, leading to an asymmetrical shape.
Comparison with Trigonal Planar Geometry
- Trigonal planar geometry involves three bonding pairs with no lone pairs, forming a flat, triangular shape.
- Trigonal pyramidal geometry includes a lone pair, creating a three-dimensional pyramidal structure.
These comparisons illustrate the unique features of trigonal pyramidal geometry and its significance in molecular studies.
Factors Influencing Trigonal Pyramidal Geometry
Several factors can influence the geometry of trigonal pyramidal molecules:
- Electronegativity: Differences in electronegativity between the central atom and surrounding atoms can affect electron distribution.
- Hybridization: The type of hybridization (sp³) determines the spatial arrangement of electron pairs.
- Substituents: The presence of bulky or electronegative substituents can alter the geometry.
Understanding these factors is essential for predicting and manipulating molecular shapes in chemical reactions.
Experimental Techniques for Identifying Trigonal Pyramidal Structures
Various experimental techniques can be used to identify trigonal pyramidal geometry:
- Spectroscopy: Infrared and Raman spectroscopy provide insights into bond angles and molecular vibrations.
- X-ray Crystallography: This technique determines the precise arrangement of atoms in a crystal lattice.
- Nuclear Magnetic Resonance (NMR): NMR spectroscopy reveals information about molecular structure and dynamics.
These techniques are indispensable tools for chemists studying molecular geometry.
Conclusion and Next Steps
Trigonal pyramidal geometry is a vital concept in chemistry, influencing the properties and behavior of numerous molecules. By understanding its characteristics, applications, and factors affecting its formation, chemists can better predict and control chemical reactions. This comprehensive guide has provided an in-depth look at trigonal pyramidal geometry, equipping you with the knowledge to explore this fascinating field further.
We encourage you to share your thoughts and questions in the comments section below. Additionally, feel free to explore other articles on our site for more insights into chemistry and molecular geometry. Together, let's continue to unravel the mysteries of the chemical world!